
Microns Matter® PFMEA Process
At Nissha Medical Technologies, mitigating risk is our fundamental goal.
Microns Matter® PFMEA Process
PLAN, PLAN, PLAN, EXECUTE
At Nissha Medical Technologies, mitigating risk is our fundamental goal. To achieve this goal, we’ve developed the Microns Matter best-practice risk assessment strategy. This proven process is used for the development and high-volume production of critical-to-function micro molded devices.
The Microns Matter PFMEA Process follows a Japanese-like methodology — essentially, “plan, plan, plan, execute”. The axiom “fail to plan, plan to fail” is never more accurate than when it’s applied to micro manufacturing projects. The risks associated with the failure to plan are numerous. Design re-iterations, re-assessment of manufacturing protocols, and adjustment or reworking of tooling can be catastrophic with time, cost, and the difference between project success and project failure

SUCCESSFUL MICRO MANUFACTURING IS DRIVEN BY RISK PREVENTION
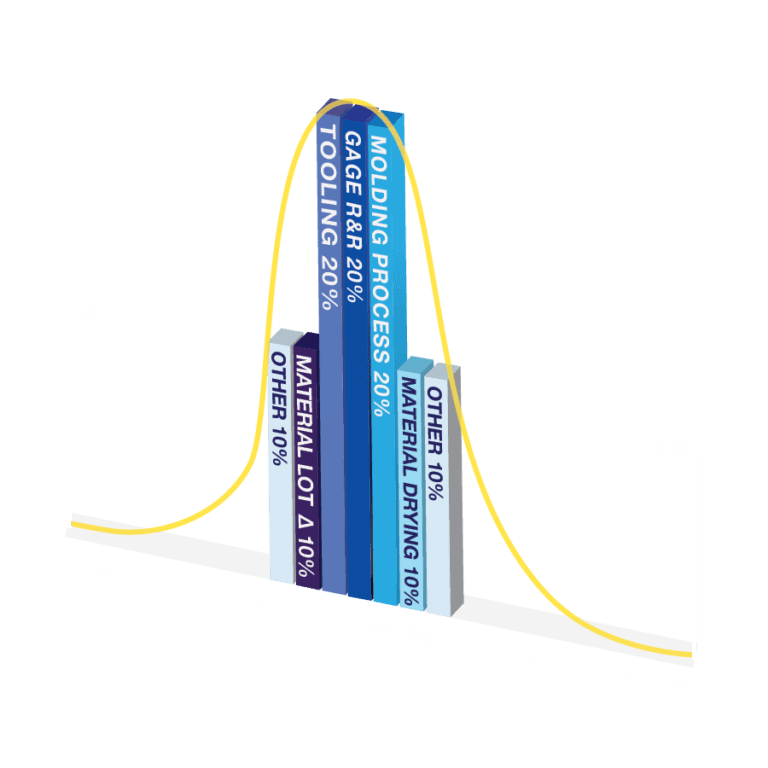
Each phase of NMT’s Microns Matter PFMEA Process allows for the accurate estimation of micron tolerance risks, which mitigates risks during each phase. Truly, components and devices with micron tolerances can only be successfully manufactured by applying years of tooling, molding, and plastics engineering experience. These phases include:
- ULTRA-PRECISION TOOLING
- METROLOGY
- MATERIAL DRYING
- MATERIAL LOT TO LOT VARIATION
- MICRO INJECTION MOLDING PROCESS
Risk prevention is driven by a complete understanding of all phases of the process used to manufacture a micro molded medical or drug delivery device. Nissha Medical Technologies will help you to get to market with optimal products in a timely fashion using our proven Microns Matter PFMEA Process.
Is the Microns Matter® Process
Right for Your Project?
If you’re asking any of these questions, your project could benefit from the Microns Matter® PFMEA Process:
- Are you and your current injection molding supplier struggling to mold or validate a small, tight-tolerance part?
- Are you unsure if your part is considered a micro molded part?
- Do you have features and tolerances on a small part that have caused you headaches?
- Would you like to see more examples of molded part material-feature-tolerance combinations that are like the parts you need?
- Have you failed with another injection molded part and need to get to the finish line of your project with a knowledgeable and transparent supplier partner?
- Do you have gaps in your design history because your micro molder is withholding valuable and critical information from you and your design team?
- Do you wish you could send a purchase order and trust that your micro parts will be shipped with first shot quality?
- Would you like to work with a fully integrated company that holds itself responsible throughout the entire process of micro mold build, micro molding, micro automation, and CT scanning, because they have ALL of these capabilities in-house?
- Would your project benefit from the Microns Matter process approach to validating medical and drug delivery components and assemblies?
- Would you like your tiny molded part or device to be automated from pellets to packout in a Class 7 cleanroom?
- Do you need a solution for a micro assembly that is automated and held in the same fixture from pellet to packout, reducing the errors in your assembled device 100-fold?
- Are you looking for a solutions-based, engineering-focused team dedicated to micron tolerance components and automated assemblies?
- Are you trying to split a plastic pellet into more than one part?
If you answered “yes” to three or more, it’s time to explore how the Microns Matter® process can help.
Tight tolerances leave
no room for guesswork.
The Microns Matter® White Paper details a data-driven process that minimizes variation and supports consistent validation outcomes.
Inside the Microns Matter® White Paper:
• How tolerance risk is distributed across tooling, molding, and metrology
• Why CT scanning replaced conventional vision inspection for micro parts
• How material behavior and humidity affect micron-level performance
Authored by Donna Bibber, with over 30 years of experience in micro injection molding and medical device manufacturing.
Ready to get Started?
We can Help
Our team is standing by to help you with your next project! Contact us today!
Global Headquarters
400 Exchange Street. Buffalo, NY 14204
P: +1.800.893.6361
E: dm.info@nisshamedical.com
E: dm.orders@nisshamedical.com

